
Zinc Zn Chemistry Zn2+ compounds oxidation state +2 complexes complex ions chemical reactions GCE AS A2 IB A level inorganic chemistry revision notes
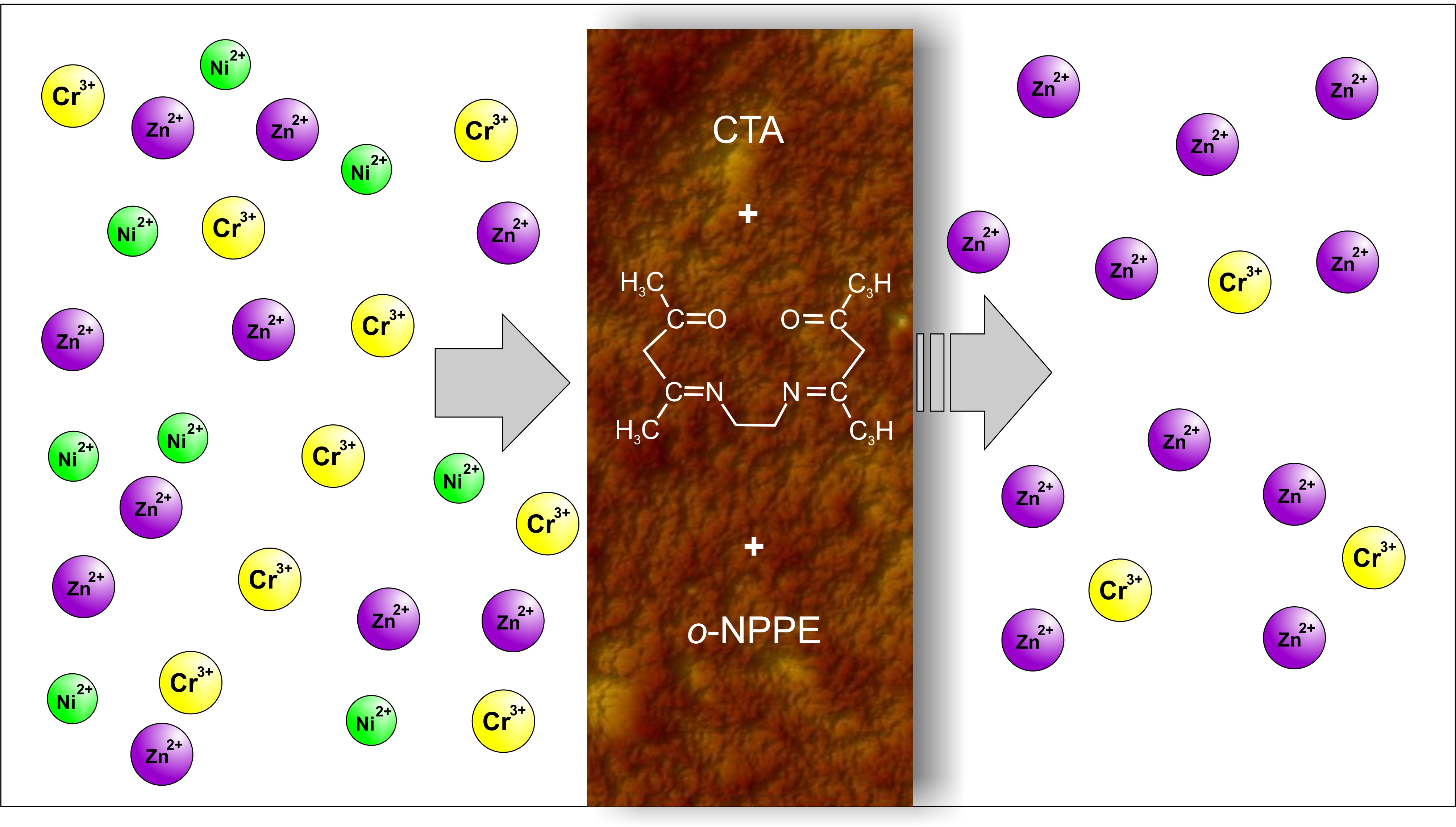
Membranes | Free Full-Text | Separation of Zn(II), Cr(III), and Ni(II) Ions Using the Polymer Inclusion Membranes Containing Acetylacetone Derivative as the Carrier

Zinc−Zinc Bonded Zincocene Structures. Synthesis and Characterization of Zn2(η5-C5Me5)2 and Zn2(η5-C5Me4Et)2 | Journal of the American Chemical Society
43. Balance the following equation: Zn + (H+) —> (Zn+2) + H2 (Zinc reacts with hydrogen ion to give Zinc ion and Hydrogen gas.)

Question Video: Describing How Oxidation Changes a Chemical Species in the Reaction between Elemental Zinc and the Lead(II) Ion | Nagwa
2, really exist? - RSC Advances (RSC Publishing) Does the compound hexaaqua-zinc(ii)bis(hydrogensulfate)dihydrate, [Zn (H2O)6](HSO4·H2O)2, really exist? - RSC Advances (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/C8RA05162C)










